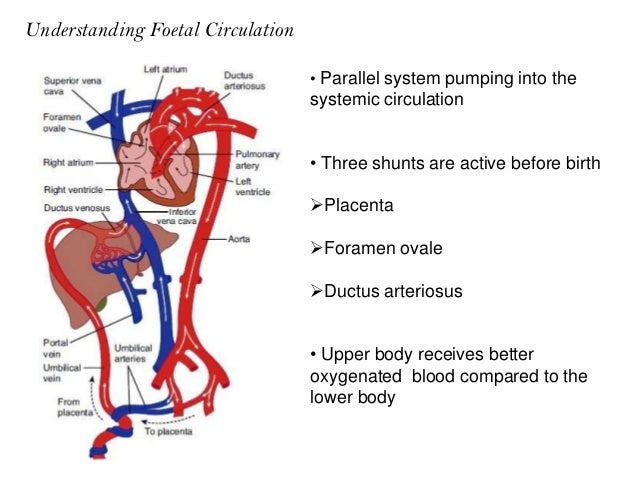
Why does the ductus arteriosis close off after birth? What is PDA in newborn? Where can you best hear PDA murmur? How is PDA diagnosed? Upon closure at birth, it becomes the ligamentum arteriosum.
Patent ductus arteriosus (PDA) is a persistent opening between the two major blood vessels leading from the heart. However, a large patent ductus arteriosus left untreated can allow poorly o. See full list on mayoclinic. A small PDA might cause no signs or symptoms and go undetected for some time — even until adulthood. A large PDA can cause signs of heart failure soon after birth. Genetic factors might play a role.
After birth, the ductus. Risk factors for having a patent ductus arteriosus include: 1. Family history and other genetic conditions. A family history of heart defects and other genetic conditions, such as Down syndrome, increase the risk of having a PDA. Rubella infection during pregnancy.

A small patent ductus arteriosus might not cause complications. Larger, untreated defects could cause: 1. High blood pressure in the lungs (pulmonary hypertension). A large patent ductus arteriosus can lead to Eisenmenger syndrome, an irreversible type of pulmonary hypertension. A patent ductus arteriosus c. Here are some of the basics: 1. Quitting smoking, reducing stress, stopping birth control — these are all things to talk to your doctor about before you get pregnant. Include a vitamin supplement that contains folic acid.
PDA (patent ductus arteriosus ) device closure surgery by Dr. In normal circulation, the right side of the baby’s heart pumps blood through the pulmonary arteries to their lungs. Ductus arteriosus, Channel between the pulmonary artery and the aorta in the fetus , which bypasses the lungs to distribute oxygen received through the placenta from the mother’s blood. It normally closes once the baby is born and the lungs inflate , separating the pulmonary and systemic circulation s. Closure before birth causes circulatory problems.
Persistent patent ductus arteriosus (PDA) is very common in preterm infants, especially in extremely preterm infants. Despite significant advances in management of these vulnerable infants, there has been no consensus on management of PDA—when should we treat, who should we treat, how should we treat and in fact there is no agreement on how we should define a hemodynamically significant PDA. In many cases, the diagnosis and treatment of a patent ductus arteriosus (PDA) is critical for survival in neonates with severe obstructive lesions to either the right or left side of the heart.
The patient presentation of patent ductus arteriosus (PDA) varies widely. Although frequently diagnosed in infants, the discovery of this condition may be delayed until childhood or even adulthood. In isolated patent ductus arteriosus (PDA), signs and symptoms are consistent with left-to-right shunting. The shunt volume is determined by the size of the open communication and the pulmonary vascular resistance (PVR). Galen initially described the ductus arteriosus in the early first century.
Harvey undertook further physiologic study in fetal circulation. Gross successfully ligated a patent ductus arteriosus (PDA) in a 7-year-old child. This was a landmark event in the history of surgery and heralded the true beginning of the field of congenital heart surgery. Premature closure of the ductus arteriosus leads to volume overload on the fetal pulmonary circulation , eventually leading to persistent pulmonary hypertension an in some cases, fetal death. Every baby is born with a ductus arteriosus.
The ductus allows blood to detour away from the lungs before birth. Failure of the ductus to close is common in premature infants but rare in full-term babies. Some children can have other heart defects along with the PDA. In a child with PDA, extra blood gets pumped from the body artery (aorta) into the lung (pulmonary) arteries. If the PDA is large, the extra blood being pumped into the lung arteries makes the heart and lungs work harder and the lungs can become congested.
Some patients can have other heart defects along with the PDA. The only abnormal finding may be a distinctive type of murmur (noise heard with a stethoscope). Infants may have trouble feeding and growing at a normal rate. High pressure may occur in the blood vessels in the lungs because more blood than normal is being pumped there. Symptoms may not occur until several weeks after birth.
Over time this may cause permanent damage to the lung blood vessels. Surgery and other treatments may not be needed. Small PDAs often close on their own within the first few months of life.
Most children can have the PDA closed by inserting catheters (long thin tubes) into the blood vessels in the leg to reach the heart and the PDA, and a coil or other device can be inserted through the catheters into the PDA like a plug. The figure below on the left shows one example of how a catheterization is used to close the ductus. If surgery is neede an incision is made in the left side of the chest, between the ribs. In premature newborn babies, medicine can often help the ductus close.
These patients may have improvement if the PDA is closed. Closing the PDA can now usually be performed by catheter coil placement or other device insertion to plug the abnormal communication (referred to as interventional or therapeutic catheterization(PDF). An incision is made in the left side of the chest, between the ribs. The PDA is closed by tying it with suture (thread-like material) or by permanently placing a small metal clip around the PDA to squeeze it closed.
Occasionally in the adult, a surgical patch is used. The long-term outlook is excellent, and usually no medicines and no additional surgery or catheterization are needed. Rarely, a patient may have a residual hole. Whether it will need to be closed depends on its size.
In a person with PDA, extra blood gets pumped from the body artery (aorta) into the lung (pulmonary) arteries. They should discuss this with their cardiologist. Only rarely will they need to take medicine after surgical or device closure. Your cardiologist can monitor you with noninvasive tests if needed. Patients with a small PDA need periodic follow-up with a cardiologist.
Exercise restriction is recommended for patients with pulmonary hypertension related to PDA. Endocarditis prophylaxis is generally not needed more than six months after PDA device closure. See the section on endocarditis for more information. It can be small or large, and accordingly, the symptoms will be mild or severe.
If the opening in the ductus arteriosus is tiny then apparently there won’t be any symptoms and the doctor will be able to determine the defect only by hearing a distant. Babies are born with a natural opening in the heart between the aorta and pulmonary artery called the ductus arteriosus. Under normal circumstances, this vessel closes naturally or becomes tiny within the first few days of life.
However, in some babies, particularly those born early (premature neonates), this hole remains open. Pathogenesis : As the ductus arteriosus closes in the fetus, blood from the right heart is totally directed to the high resistance fluid-filled lungs, resulting in increased right ventricular afterloa impaired right ventricular function, and consequently tricuspid regurgitation and right heart dilatation. Spontaneous DA closure time curves were clarified for the first time throughout the early neonatal period in full-term and appropriate for gestational age neonates. PDA gives rise to a left-to-right shunt, and allows the blood to move from systemic circulation to pulmonary circulation. Hence, there is an excess pulmonary blood flow.
In a catheter procedure, a thin tube (catheter) is inserted into a blood vessel in the groin and threaded up to the heart. Through the catheter, a plug or coil is inserted to close the ductus arteriosus. This hypoxic zone is associated with local induction of smooth muscle cell death in the media and local production of hypoxia-inducible growth factors.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.